Over the past decade the Bee population has been steadily declining. Colony Collapse Disorder has been noted world wide, with no explanation until recent. some thought that the magnetic field of the earth and the Ozone layer had to do with the insects being directionally hindered by their own biological navigation systems. recently it has been found that, Acetylcholinesterase by way of external intoxicants have been the cause of these abnormal behaviors. Acetylcholinesterase is defined as: "encoded by HGNC gene ACHE; EC 3.1.1.7 is the primary cholinesterase in the body. It is an enzyme that catalyzes the breakdown of acetylcholine and of some other choline esters that function as neurotransmitters". This process has been studied in relation to neonictonoids, glyphosates and atrazine, covered in source (1)
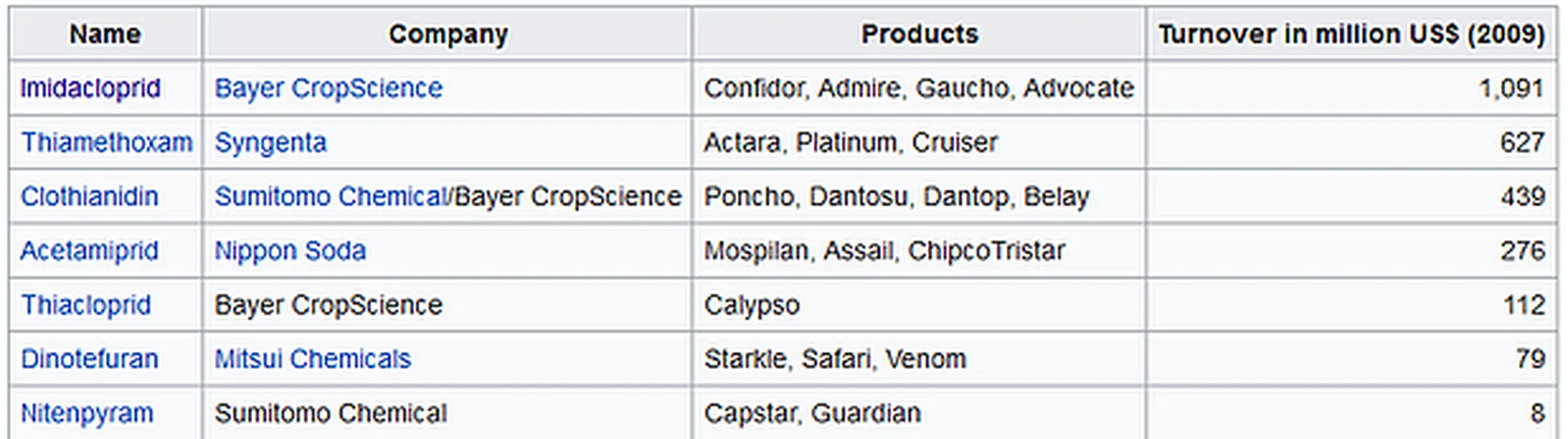
Bayers pesticide formula contains neonictonoids; "neonicotinoids, (represented as imidacloprid) cause less toxicity in birds and mammals than insects. Some breakdown products are also toxic to insects" (2) The specific neonictoinoid that Bayer produces is imidacloprid depicted in the figure below.
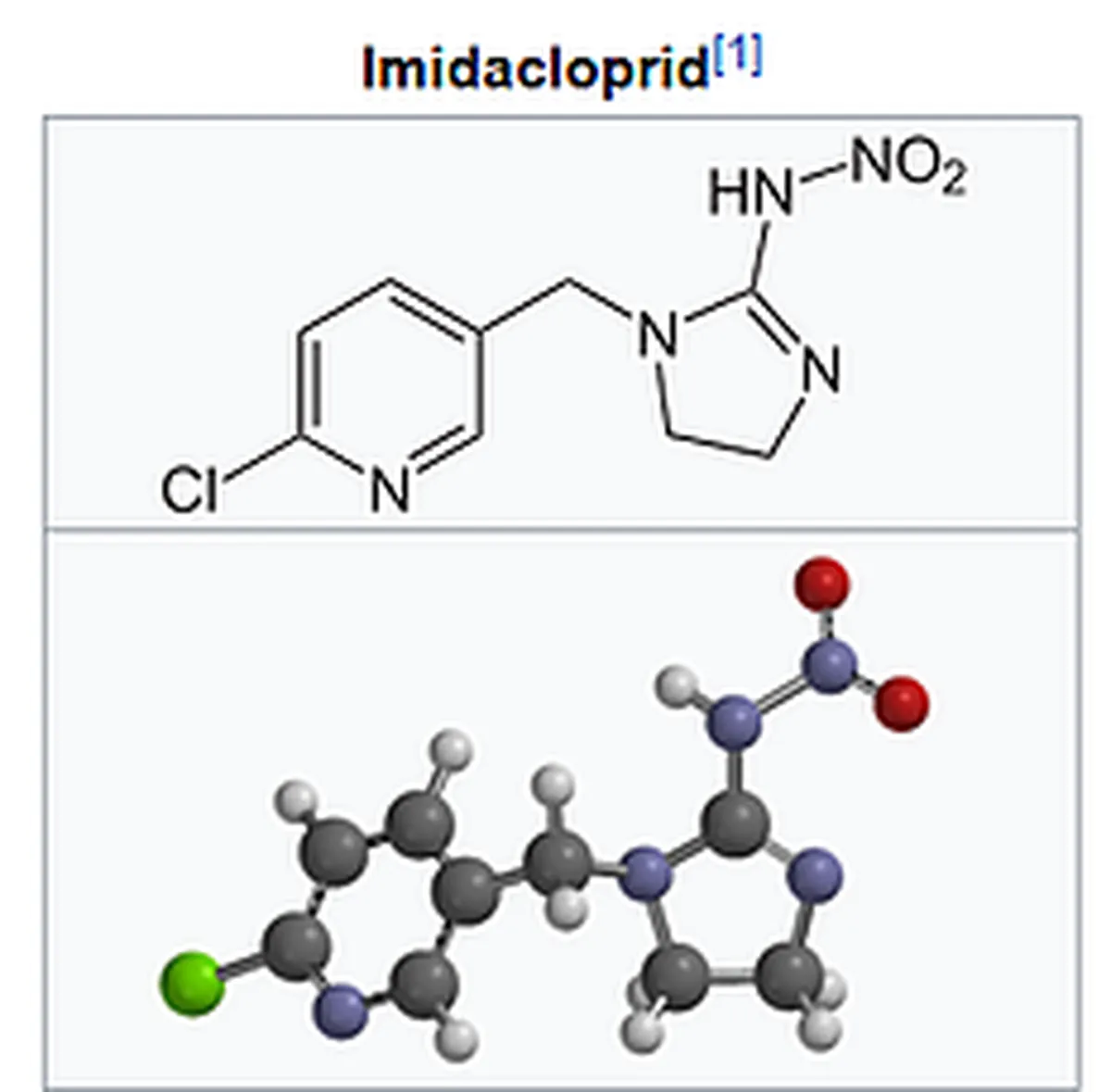
Monsantos pesticide formula contains Glyphosate; "Monsanto brought it (glyphosates) to market for agricultural use in 1974 under the trade name Roundup. Monsanto's last commercially relevant United States patent expired in 2000" (3) also depicted in the figure below.
In light of a recent lawsuit in which a janitor successfully sued Monsanto for claims that their product roundup caused cancer, Monsanto ultimately sold their company to save face, Bayer is the company that purchased them. It is a very fitting chain of events.Because bees are our polinators, their extinction requires human intervention to maintain the current ecosystem; something that should have never happened under any circumstance. The development of autonomous polinators to replace the mechanism implemented by the honey bees has been patented by Wal-Mart as "Systems and methods for dispensing pollen onto crops via unmanned vehicles"
Found below is information pertaining to the chemicals that Monsanto and Bayer have implemented as well as their structure and composition.
Sources
- Monsanto developed and patented the use of glyphosate to kill weeds in the early 1970s and first brought it to market in 1974 under the Roundup brandname.[27][28] While its initial patent[29] expired in 1991, Monsanto retained exclusive rights in the United States until its patent[30] on the isopropylamine salt expired in September 2000.[31] In 2008, scientists at the United States Department of Agriculture Agricultural Research Service (USDA ARS) described glyphosate as a 'virtually ideal' herbicide.[27] In 2010 Powles stated: 'glyphosate is a one in a 100-year discovery that is as important for reliable global food production as penicillin is for battling disease.'[32] As of April 2017, the Canadian government stated that glyphosate was 'the most widely used herbicide in Canada',[33] at which date the product labels were revised to ensure a limit of 20% POEA by weight.[33][failed verification] Health Canada's Pest Management Regulatory Agency found no risk to humans or the environment at that 20% limit, and that all products registered in Canada at that time were at or below that limit
- Neonicotinoids, like nicotine, bind to nicotinic acetylcholine receptors (nAChRs) of a cell and trigger a response by that cell. In mammals, nicotinic acetylcholine receptors are located in cells of both the central nervous system and peripheral nervous systems. In insects, these receptors are limited to the central nervous system. Nicotinic acetylcholine receptors are activated by the neurotransmitter acetylcholine. While low to moderate activation of these receptors causes nervous stimulation, high levels overstimulate and block the receptors,[5][35] causing paralysis and death. Acetylcholinesterase breaks down acetylcholine to terminate signals from these receptors. However, acetylcholinesterase cannot break down neonicotinoids and their binding is irreversible.[35] Neonicotinoids were assigned to IRAC group 4A
- In Québec, as observed globally, abnormally high honey bee mortality rates have been reported recently. Several potential contributing factors have been identified, and exposure to pesticides is of increasing concern. In maize fields, foraging bees are exposed to residual concentrations of insecticides such as neonicotinoids used for seed coating. Highly toxic to bees, neonicotinoids are also reported to increase AChE activity in other invertebrates exposed to sub-lethal doses. The purpose of this study was therefore to test if the honey bee’s AChE activity could be altered by neonicotinoid compounds and to explore possible effects of other common products used in maize fields: atrazine and glyphosate. One week prior to pollen shedding, beehives were placed near three different field types: certified organically grown maize, conventionally grown maize or non-cultivated. At the same time, caged bees were exposed to increasing sub-lethal doses of neonicotinoid insecticides (imidacloprid and clothianidin) and herbicides (atrazine and glyphosate) under controlled conditions. While increased AChE activity was found in all fields after 2 weeks of exposure, bees close to conventional maize crops showed values higher than those in both organic maize fields and non-cultivated areas. In caged bees, AChE activity increased in response to neonicotinoids, and a slight decrease was observed by glyphosate. These results are discussed with regard to AChE activity as a potential biomarker of exposure for neonicotinoids
- Glucosinolate breakdown products are volatile, therefore good candidates for insect fumigants. However, although they are insecticidal, the mode of action of such natural products is not clear. We studied the insecticidal effect of these compounds as fumigants, and monitored the production of carbon dioxide by the insects as a probe to the understanding of their mode of action. The fumigation 24-h LC50 against the house fly (Musca domestica L.) of allyl thiocyanate, allyl isothiocyanate, allyl cyanide, and l-cyano-2-hydroxy-3-butene was 0.1, 0.13, 3.66, and 6.2 μg cm-3, respectively; they were 0.55, 1.57, 2.8, and > 19.60 μg cm-3, respectively, against the lesser grain borer (Rhyzopertha dominica Fabricius). The fumigation toxicity of some of the glucosinolate products was very close to or better than that of the commercial insect fumigants such as chloropicrin (LC50: 0.08 and 1.3 μg cm-3 against M. domestica and R. dominica, respectively) and dichlorovos (LC50: < 0.02 and 0.29 μg cm-3 against M. domestica and R. dominica, respectively) in our laboratory tests. Significantly increased CO2 expiration was found in insects exposed to the vapor of allyl isothiocyanate, allyl thiocyanate and allyl isocyanate. Allyl isothiocyanate was also found to increase the CO2 expiration of the American cockroach (Periplaneta americana L.). Glucosinolate breakdown products have potential as biodegradable and safe insect fumigants. They may act on the insect respiratory system in their mode of action




